Infectious Disease
EIA Kits

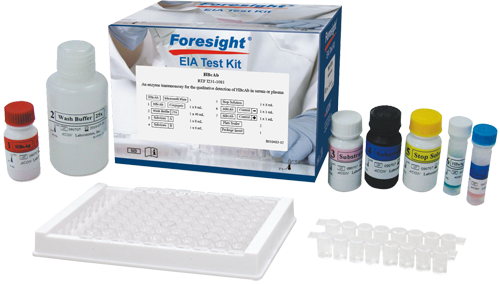
Product is Available for International Distribution Only – Not Available in the US
Foresight® Infectious Disease EIA Kits
SARS-CoV-2 IgG and IgM
The SARS-CoV-2 IgG and IgM EIA Test Kits are qualitative enzyme immunoassays for the detection of IgG or IgM antibodies to SARS-CoV-2 in human serum or plasma.*

High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance at competitive pricing
Simple and Easy to Use
- Total incubation time as short as 70 minutes
- Simplified procedure designed for ease of use
- Color-coded and numbered reagents for easy handling
- Ready to use reagents shorten initial preparation
Convenient
- All necessary reagents provided
- Breakaway 8-well strips in 96 microwell plate in resealable foil pouch
- Can be performed on automated sample processors
- Shelf life up to 12 months
CE Marked for sale in the European Community
Product Specifications
| Feature | SARS-CoV-2 IgG | SARS-CoV-2 IgM |
| Catalog No. | I231-1321 | I231-1331 |
| Tests per Kit | 96/480 | 96/480 |
| Detection | IgG antibodies to SARS-CoV-2 | IgM antibodies to SARS-CoV-2 |
| Type of Test | Qualitative | Qualitative |
| Principle | Indirect EIA | Capture EIA |
| Specimen Type | Serum/Plasma | Serum/Plasma |
| Specimen Volume | 5 μL | 5 μL |
| Specimen Diluent Volume | 100 μL | 100 μL |
| Neutralization Solution Volume | N/A | N/A |
| Conjugate Volume | 100 μL | 100 μL |
| Substrate A/B Volume | 50 μL/50 μL | 50 μL/50 μL |
| Stop Solution Volume | 50 μL | 50 μL |
| Total Incubation Time | 70 minutes (30/30/10) | 70 minutes (30/30/10) |
| Wavelength | 450/630-700 nm | 450/630-700 nm |
| Sensitivity | >99.9% (95% CI: 92.1%~100%) | 88.6% (95% CI: 76.0%~95.0%) |
| Specificity | 99.4% (95% CI: 96.9%~100%) | 97.8% (95% CI: 94.6%~99.2%) |
| Overall Agreement | 99.6% (95% CI: 97.5%~100%) | 96.1% (95% CI: 92.7%~97.9%) |
| Within-Run Precision CV (%) | <9% | <12% |
| Between-Run Precision CV (%) | <15% | <13% |
| Shelf Life | 12 months | 12 months |
* The SARS-CoV-2 IgG and IgM EIA Test Kits are only intended for assisting detection of suspected cases with nucleic acid negative, or combining with nucleic acid detection in the diagnosis of suspected cases. Results from SARS-CoV-2 IgG and IgM testing should not be used as the sole basis to diagnosis or exclude SARS-CoV-2 infection.
H. pylori Antigen
Qualitative and quantitative detection of Helicobacter pylori (H. pylori) Antigen in stool
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Room temperature incubation
- Total incubation time: 70 min.
Convenient
- Specimen collection device included in kit
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 18 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1231 |
| Tests per Kit | 96 |
| Detection | Helicobacter pylori (H. pylori) Antigen |
| Type of Test | Qualitative/Quantitative |
| Principle | “Sandwich” EIA |
| Specimen | Stool |
| Specimen Volume | Solid: 30 mg; Liquid: 50 μL |
| Extraction solution volume | 1000 μL |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 70 min (60/10 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 98.6% |
| Specificity | 95.4% |
| Overall Agreement | 96.5% |
H. pylori IgG
Qualitative and quantitative detection of IgG antibodies to Helicobacter pylori (H. pylori) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Color change confirms specimen has been added
- Total incubation time: 70 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 18 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1241 |
| Tests per Kit | 96 |
| Detection | IgG antibodies to Helicobacter pylori (H. pylori) |
| Type of Test | Qualitative/Quantitative |
| Principle | Indirect EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 5 μL |
| Specimen Diluent Volume | 100 μL |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 100 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 70 min (30/30/10 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 97.9% |
| Specificity | >99.9% |
| Overall Agreement | 98.6% |
HBcAb
Qualitative detection of Hepatitis B Core Antibody (HBcAb) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 45 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1081 |
| Tests per Kit | 96/480 |
| Detection | Hepatitis B Core Antibody |
| Type of Test | Qualitative |
| Principle | Competitive EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 45 min (30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.3% |
| Specificity | 99.4% |
| Overall Agreement | 99.3% |
HBeAb
Qualitative detection of Hepatitis B Envelope Antibody (HBeAb) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 45 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1071 |
| Tests per Kit | 96/480 |
| Detection | Hepatitis B Envelope Antibody including IgG,IgA, IgM |
| Type of Test | Qualitative |
| Principle | Competitive EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | 50 μL |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 45 min (30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.1% |
| Specificity | 99.5% |
| Overall Agreement | 99.4% |
HBeAg
Qualitative detection of Hepatitis B Envelope Antigen (HBeAg) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 45 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1061 |
| Tests per Kit | 96/480 |
| Detection | Hepatitis B Envelope Antigen |
| Type of Test | Qualitative |
| Principle | Double antibody “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 45 min (30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.7% |
| Specificity | 99.8% |
| Overall Agreement | 99.8% |
HBsAb
Qualitative detection of Hepatitis B Surface Antibody (HBsAb) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 45 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1051 |
| Tests per Kit | 96/480 |
| Detection | Hepatitis B Surface Antibody |
| Type of Test | Qualitative |
| Principle | Double antigen “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 45 min (30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.3% |
| Specificity | 99.1% |
| Overall Agreement | 99.2% |
HBsAb Quantitative
Quantitative detection of Hepatitis B Surface Antibody (HBsAb) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 45 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1271 |
| Tests per Kit | 96/480 |
| Detection | Hepatitis B Surface Antibody |
| Type of Test | Quantitative |
| Principle | Double antigen “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 45 min (30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.3% |
| Specificity | 99.2% |
| Overall Agreement | 99.2% |
HBsAg
Qualitative detection of Hepatitis B Surface Antigen (HBsAg) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 70 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 18 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1021 |
| Tests per Kit | 96/480 |
| Detection | Ad and ay subtypes of Hepatitis B surface antigens |
| Type of Test | Qualitative |
| Principle | Double antibody “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 100 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | Standard Procedure: 70 min (60/10 min) Enhanced Procedure: 150 min (120/30 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | Standard Procedure: 0.2 IU/mL Enhanced Procedure: 0.1 IU/mL |
| Sensitivity | >99.9% |
| Specificity | 99.9% |
| Overall Agreement | 99.9% |
HCV
Qualitative detection of IgG antibodies to Hepatitis C Virus (HCV) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Color change confirms specimen has been added
- Total incubation time: 70 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 21 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1031 |
| Tests per Kit | 96/480 |
| Detection | HCV IgG antibodies using recombinant antigens specific for core, NS3, NS4, and NS5 |
| Type of Test | Qualitative |
| Principle | Indirect EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 10 μL |
| Specimen Diluent Volume | 100 μL |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 100 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 70 min (30/30/10 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | >99.9% |
| Specificity | 99.8% |
| Overall Agreement | 99.8% |
HEV IgM
Qualitative detection of Hepatitis E IgM antibodies in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Color change confirms specimen has been added
- Room temperature incubation
- Total incubation time: 70 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 15 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1211 |
| Tests per Kit | 96 |
| Detection | Hepatitis E IgM antibodies |
| Type of Test | Qualitative |
| Principle | Immunocapture EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 10 μL |
| Specimen Diluent Volume | 100 μL |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 100 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 75 min (30/30/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | 99.2% |
| Specificity | 99.6% |
| Overall Agreement | 99.6% |
HIV 1/2/O Ab
Qualitative detection of total antibodies (IgG, IgM, and IgA) to Human Immunodeficiency Virus (HIV) type 1 and type 2, and/or Subtype O in human serum or plasma

High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 60 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 18 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1011 |
| Tests per Kit | 96/480 |
| Detection | HIV-1, HIV-2, and/or Subtype O total antibodies |
| Type of Test | Qualitative |
| Principle | Double antigen “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 100 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 100 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 60 min (30/20/10 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | >99.9% |
| Specificity | 99.8% |
| Overall Agreement | 99.8% |
HTLV I/II
Qualitative detection of antibodies to HTLV I/II in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 90 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 12 months
Product Specifications
| Feature | Specifications |
| Catalog No. | I231-1291 |
| Tests per Kit | 96/480 |
| Detection | HTLV I/II antibodies |
| Type of Test | Qualitative |
| Principle | Double antigen “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 90 min (30/30/30 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | >99.9% |
| Specificity | >99.9% |
| Overall Agreement | 99.9% |
Syphilis
Qualitative detection of total antibodies (IgG, IgM and IgA) to Treponema Pallidum (TP) in serum or plasma
High Quality
- High sensitivity and specificity
- High reproducibility
- Consistent and reliable performance
Easy to Use
- Standardized, color-coded and numbered reagents
- Ready-to- use reagents shorten initial prep time
- Total incubation time: 75 min.
Convenient
- All necessary reagents provided
- Breakaway 8-well strips / 96-microwell plate in re-sealable foil pouch
- Shelf life of 18 months
Product Specifications
| Feature | Specification |
| Catalog No. | I231-1041 |
| Tests per Kit | 96/480 |
| Detection | Syphilis IgG, IgM, and IgA antibodies using recombinant antigens specific for TP15, TP17, TP47 |
| Type of Test | Qualitative |
| Principle | Double antigen “sandwich” EIA |
| Specimen | Serum/Plasma |
| Specimen Volume | 50 μL |
| Specimen Diluent Volume | N/A |
| Neutralization Solution Volume | N/A |
| Conjugate Volume | 50 μL |
| Substrate A/B Volume | 50 μL/50 μL |
| Stop Solution Volume | 50 μL |
| Total Incubation Time | 75 min (60/15 min) |
| Wavelength | 450 or 450/630-700 nm |
| Analytical Sensitivity | N/A |
| Sensitivity | >99.9% |
| Specificity | 99.8% |
| Overall Agreement | 99.8% |
Resource Library
Browse our Resources Library for documents available for download.
