Flowflex®
COVID-19 Antigen Home Test
A rapid test for the detection of COVID-19 antigens in nasal specimens.

ACON Laboratories, Inc. is the only legal manufacturer of the FDA EUA Flowflex COVID-19 Antigen Home Test.
Critical Information When & Where You Need It
- Affordable
- Easy-to-Use Nasal Swab Test
- Results in 15 minutes
- Safe for children as young as 2 years old
- For use with and without symptoms

Expiration Date Extension
As of March 15, 2023, the expiration date of the Flowflex COVID-19 Antigen Home Test has been extended by 12 months. Please click here to check the new expiration date using the lot number printed on the test kit box.
Test Procedure Overview
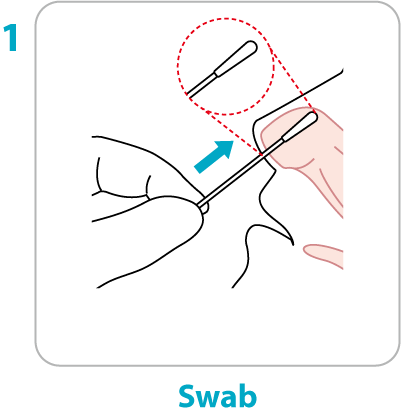
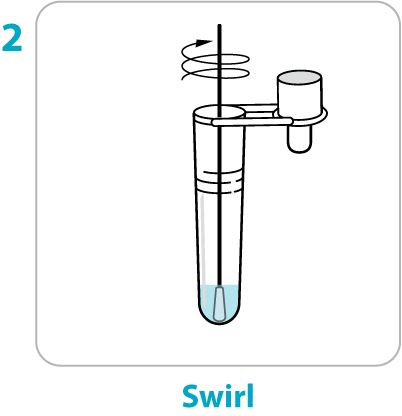
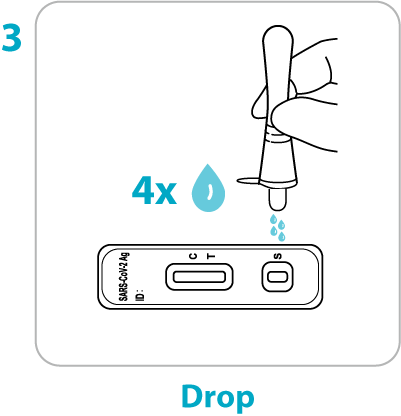

This test procedure overview does not replace the package insert. Before you begin the test, it is important to read and follow the detailed instructions in the package insert.
Resource Library
Flowflex® COVID-19 Antigen Home Test documents available for download.
Flowflex Covid-19 Antigen Home Test Consumer Package Insert
Flowflex Covid-19 Antigen Home Test Consumer Package Insert (Spanish)
Flowflex Covid-19 Antigen Home Test Fact Sheet for Healthcare Professionals
Flowflex Covid-19 Antigen Home Test Healthcare Provider Package Insert
Flowflex Covid-19 Antigen Home Test Distributor Sell Sheet
Flowflex Covid-19 Antigen Home Test Quick Reference Instructions (English)
Flowflex Covid-19 Antigen Home Test Quick Reference Instructions (Spanish)
Frequently Asked Questions
- In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19